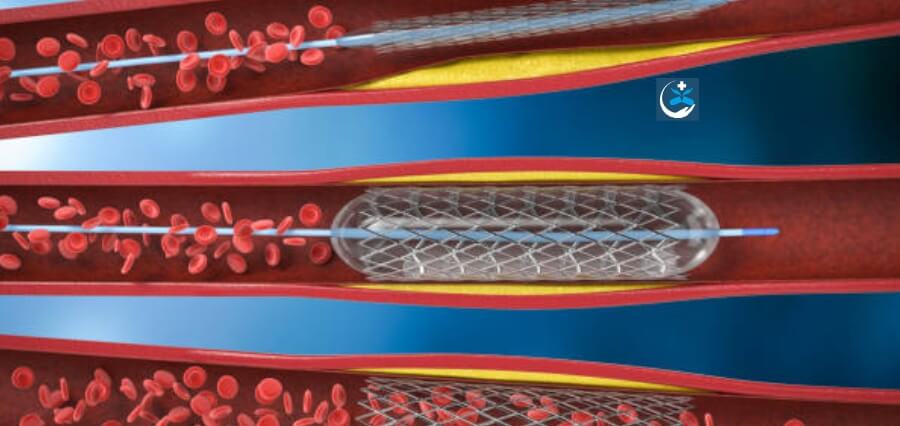
Irish medical device company Ceroflo revealed today that it has secured €6.4 million in investment-round funding. The funds are earmarked for advancing the development of the company’s SubMax device, a groundbreaking stent designed to transform treatments for intracranial atherosclerotic disease (ICAD). According to a press release from Ceroflo, these funds will facilitate the initiation of first-in-human clinical trials for its innovative stroke technology, involving 30 patients.
Ceroflo enlisted accounting firm DHKN to spearhead an Employment and Investment Incentive Scheme (EIIS) investment round. Within a few weeks, the round garnered €5 million, with an extra €1.4 million secured from “highly respected Irish MedTech entrepreneurs and prominent global stroke key opinion leaders,” as stated in the company’s recent announcement.
Ceroflo asserts that approximately 10–15% of strokes are attributed to ICAD, for which existing treatment choices, such as pharmaceutical therapies, are deemed “suboptimal.” Consequently, patients are left with a persistent risk of experiencing a stroke.
The company asserts that its SubMax stent represents a groundbreaking advancement in the treatment of ICAD. Its shape and structure have been meticulously developed to address the unique challenges posed by this disease. The stent is engineered to gently enhance critical blood flow to the brain while mitigating the risks associated with first-generation devices, such as hemorrhage and stroke.
In November of last year, a consortium led by Ceroflo, which includes manufacturing firm Advant Medical and the Medical and Engineering Technologies Centre at Atlantic Technological University in Galway, Ireland, secured €3.4 million from the Irish Department of Enterprise, Trade, and Employment’s Disruptive Technologies Innovation Fund (DTIF).
Situated in Galway City, Ireland, Ceroflo assembles a team comprising professionals from the Irish medical device industry. This team includes its co-founder and chairman, Eamon Brady; co-founder John O’Dea; CEO Chloe Brown; Chief Technology Officer Brendan Casey; and advisor John O’Shaughnessy. Additionally, other company co-founders encompass prominent stroke interventionists Tommy Andersson (Karolinska University Hospital, Stockholm, Sweden), Leonard Yeo (National University of Health Singapore, Singapore), and Paul Bhogal (Royal London Hospital, London, UK).
“Ceroflo is pioneering the development of an innovative stent device to tackle ICAD, representing the next frontier in stroke treatment and prevention,” stated CEO Brown. “We approach this challenge with a unique understanding of its complexities and collaborate closely with clinicians boasting over 50 years of combined experience in treating this condition. This marks an exceptionally positive period for Ceroflo. Building on the success of our DTIF grant award and the remarkable progress made over the past year, we are enthusiastic about advancing the technology further. With this €6.4 million investment, Ceroflo will conduct a first-in-human trial, involving 30 patients, to assess the SubMax stent—a pivotal milestone. Furthermore, it will provide us with the resources to facilitate additional regulatory studies in the US and Japan.”