All adults with type 2 diabetes or prediabetes should be evaluated for non-alcoholic fatty liver disease, an increasingly common condition that can cause catastrophic liver damage, according to the American Diabetes Association on Sunday.
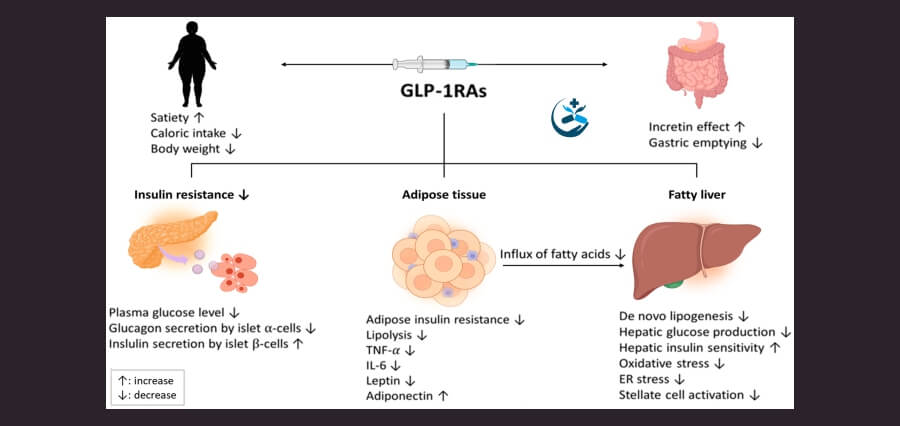
The ADA singled out GLP-1 treatments as a diabetes pharmacological alternative that clinicians could take into consideration, according to recommendations presented during the annual ADA conference. There are no approved medications for the condition.
GLP-1 medications, including Ozempic and Mounjaro, are a class of medications that have gained a lot of popularity for their effectiveness in reducing weight in addition to blood sugar levels. The pharmaceutical industry has begun to research these drugs for liver illness, and while some studies have suggested they may have some advantages, they have not yet been demonstrated to reduce dangerous liver scarring.
According to Robert Gabbay, the ADA’s chief science and medical officer, “we realised this is just becoming such a pervasive issue” and as a result, the organisation produced recommendations on the illness. “Type 2 diabetes and obesity are increasingly the main contributors to liver disease, but that’s not really on people’s minds when they think about diabetes,”
In the United States, non-alcoholic fatty liver disease, which develops when too much fat accumulates in the liver, is thought to afflict roughly 24% of adults. Nonalcoholic steatohepatitis, or NASH, is a rarer, more severe form of the disease that causes liver inflammation and scarring. It is now one of the main reasons for liver transplants and liver cancer.
Despite the condition’s rising incidence, no medications have been approved to treat it. The Food and Drug Administration recently rejected a NASH medication from Intercept Pharmaceuticals due to safety concerns raised by agency advisers.