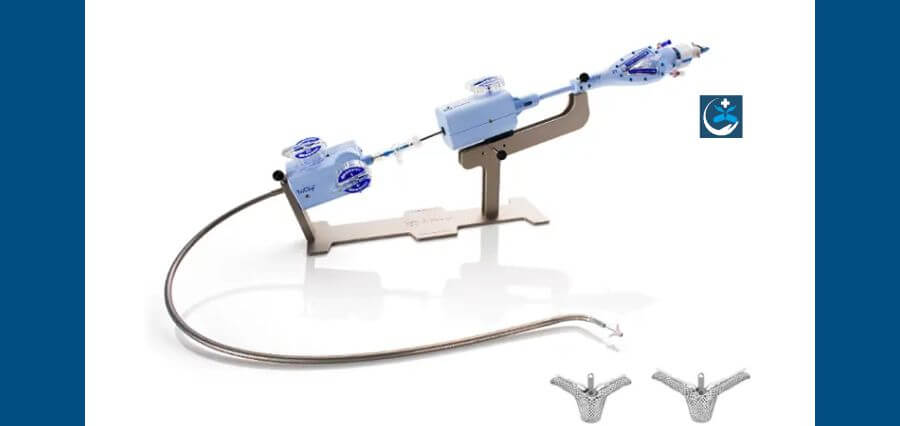
Abbott Laboratories (ABT.N) has acquired FDA approval for its heart valve repair device for patients with potentially deadly cardiac disease. This comes just months after Edwards Lifesciences (EW.N) received the same approval.
The Abbott device, TriClip, is intended to treat tricuspid regurgitation (TR), which occurs when the valve that separates the right lower chamber of the heart from the right upper chamber fails to shut properly, causing blood to flow backward.
The syndrome primarily affects older people with many co-morbidities, such as irregular heartbeat and high blood pressure in the lungs or heart, who are at high risk of complications or death from open-heart surgery.
According to government estimates, the illness affects approximately 1.6 million Americans.
In February, Edwards Lifesciences received approval to replace the tricuspid valve. Unlike Edwards’ competition, Abbott’s gadget fixes the tricuspid valve by removing a piece of its flaps to reduce blood backflow.
Other treatment options for the condition include diuretic medicines, which cause patients to urinate regularly to reduce fluid buildup in the body.
“The key message to remember here is that patients now have options,” said Nadim Geloo, Senior Director of Medical Affairs for Abbott’s Structural Heart Division.
Geloo stated that mending the valve rather than replacing it is a “very positive treatment path,” and that 98% of patients who had TriClip saw no serious side effects within 30 days.
Abbott included TriClip, which is already approved in more than 50 countries, in its group of “Fab 5” products, which are projected to drive sales growth in the coming years.
The gadget is injected into the leg’s femoral vein before being guided and clipped to the tricuspid valve.